Women live longer than men. This isn’t unique to humans, either; we see this trend in a wide range of other animals. Biologists have theorized that the discrepancy in life expectancy between sexes might be partly related to reproduction, but how?
In a study published in Science Advances, researchers from Osaka University have discovered for the first time that germ cells, the cells that develop into eggs in females and sperm in males, drive sex-dependent lifespan differences in vertebrate animals.
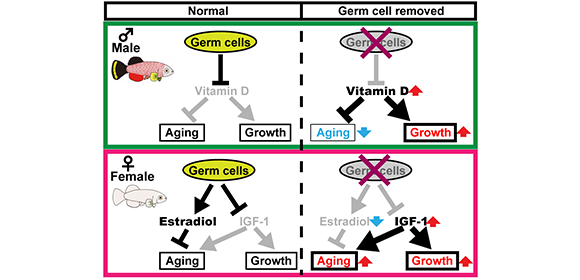
The researchers examined aging in the turquoise killifish, a small, fast-growing freshwater fish with a lifespan of only a few months. As in humans, female killifish live longer than males. However, when the researchers removed the germ cells from these fish, they found that males and females had similar lifespans.
“After removing the germ cells, male killifish lived longer than usual and female lifespans became shorter,” explains lead author Kota Abe. “We wanted to understand how germ cells could affect males and females so differently. Our next step was to investigate the factors responsible.”
The team found that hormonal signaling was very different in females than in males. Female killifish without germ cells had significantly less estrogen signaling, which can shorten lifespan by increasing cardiovascular disease risk. The females also had significantly more growth factor signaling (insulin-like growth factor 1). This made the females grow larger while also suppressing signals within the body important for maintaining health and slowing aging.
In contrast, male killifish without germ cells had improved muscle, skin, and bone health. Interestingly, these fish had increased amounts of a substance that activates vitamin D, as well as evidence of vitamin D signaling in their muscles and skin.
Vitamin D can also be considered a hormone; while well known for keeping bones strong and healthy, it also seems to have wider positive effects throughout the body. The team’s results pointed to the possibility that vitamin D can improve longevity, leading them to test whether a vitamin D supplement could increase the lifespan of the fish.
“When we administered active vitamin D, we found that the lifespans of both males and females were significantly extended, suggesting that vitamin D signaling provides health benefits throughout the body,” explains senior author Tohru Ishitani. “Our work suggests that vitamin D signaling could influence the longevity of other vertebrates, including humans.”
The discovery that germ cells affect male and female longevity in opposing ways is an important clue in unravelling the mysterious interactions between reproduction, aging, and lifespan. It’s unclear exactly how vitamin D fits into this puzzle, but it could be part of future strategies to extend healthy lifespans.
Fig. 1
Model of sex-dependent regulation of organismal aging in vertebrates by germ cells.
Credit: Osaka University
Fig. 2
Germ-cell-removal shortens the lifespan in females, but extends that in males.(A) Germ-cell-removed turquoise killifish and their gonads. (B) Lifespan of germ-cell-removed animals.
Credit: 2024 Ishitani et al., Sex-dependent regulation of vertebrate somatic growth and aging by germ cells, Science Advances
Fig. 3
Treatment of vitamin D extends the lifespan in both sexes.
Credit: 2024 Ishitani et al., Sex-dependent regulation of vertebrate somatic growth and aging by germ cells, Science Advances