This image shows a hibernating little brown bat infected with white-nose syndrome. Photo credit: Jonathan Mays
An invasive fungus that colonizes the skin of hibernating bats with deadly consequences is a stealthy invader that uses multiple strategies to slip into the small mammals’ skin cells and quietly manipulate them to aid its own survival. The fungus, which causes the disease white-nose syndrome, has devastated several North American species over the last 18 years.
Scientists have learned much about the fungus, Pseudogymnoascus destructans, since it was first documented in a New York cave in 2006, including where it thrives, its distribution, and clinical features. But exactly how the fungus initiates its infection has remained a “black box – a big mystery,” says Bruce Klein, a professor of pediatrics, medicine and medical microbiology and immunology at the University of Wisconsin-Madison.

Bruce Klein
That dearth of understanding has made it challenging to develop countermeasures to treat or prevent infections.
Now, Klein and Marcos Isidoro-Ayza, a PhD candidate at Klein’s lab, have for the first time been able to study in detail how the fungus gains entry and covertly hijacks cells called keratinocytes at the surface of bats’ skin.
The feat is detailed in the July 12, 2024, issue of Science.
The researchers found that P. destructans uses infected cells as a refuge and prevents the cells from dying, which in turn thwarts the bats’ immune system and allows the microbial invader to continue growing and slip into more cells.

Marcos Isidoro-Ayza
To do so, Klein and Isidoro-Ayza created the first-ever keratinocyte cell line from the skin of a little brown bat. Further, they successfully mimicked the conditions of hibernation, which are marked by wide body temperature fluctuations that accompany periods of torpor – when the animals’ metabolism slows and body temperature drops – and arousal.
This is crucial to understanding P. destructans infections because the cold-loving fungus gains its foothold during the chilly conditions of torpor and is able to persist during arousal, when the bats’ body temperature increases.
Klein and Isidoro-Ayza have already identified how the fungus gains its stealth entry into cells: by co-opting a protein on their surface called epidermal growth factor receptor, or EGFR. Mutations in the same receptor in human cells drive certain lung cancers and these cancers are treated with an existing drug called gefitinib, opening the possibility it could be used to treat or prevent white-nose syndrome.
“Remarkably, when we inhibited the receptor with this drug, we stopped the infection,” says Isidoro-Ayza. “This is an FDA-approved drug that could potentially be used in the future for the treatment of susceptible bat species.”
While EGFR’s precise role in infection is not yet completely clear, Klein and Isidoro-Ayza have learned much about how the fungus works.
Its initial entry occurs during torpor, when bats’ immune systems are dormant and their body temperature is in an ideal range for P. destructans to germinate and grow. During torpor, the fungus penetrates bats’ skin cells with its hyphae – slender filaments through which it grows and gathers nutrients – without breaking the cells’ membranes. Doing so would trigger the cells’ death and expose the fungus to the bats’ immune system.
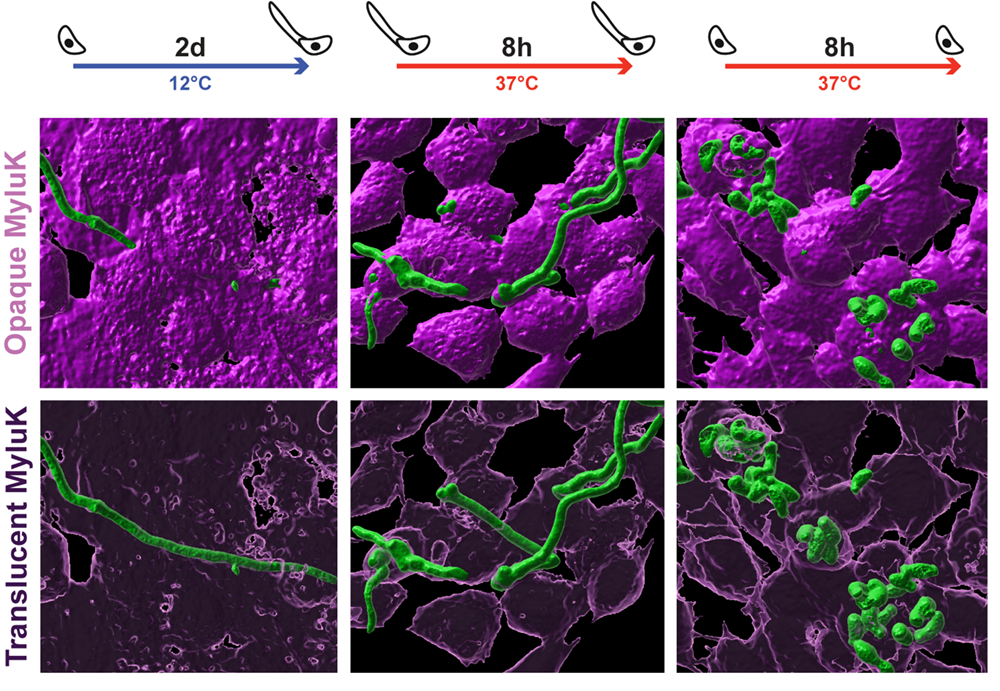
These images show the hyphae and spores of the fungus that causes white-nose syndrome (in green) invading the lab grown skin cells of little brown bats. Credit: Klein Lab / UW-Madison
Klein and Isidoro-Ayza also found that the fungus employs multiple strategies that allow it to continue its invasion during periods of arousal, despite the bats’ higher body temperatures and reactivated immune system.
First, during arousal periods, the fungus manipulates the cells so they engulf the fungus in a process known as endocytosis, rather than the fungus using its hyphae to penetrate the cell.
Second, they found that the fungus spores – microscopic particles by which it reproduces – are coated in a layer of melanin that protects them from the cells’ strategies for killing invading microbes.
“That allows the spore to survive that period of arousal, and when the bat goes back into torpor, the spores inside of its cells start germinating again and keep colonizing the skin,” explains Isidoro-Ayza, who is a student in the UW-Madison School of Veterinary Medicine’s comparative biomedical sciences graduate program.
P. destructans’ final infection strategy is to block apoptosis, also known as programmed cell death, which is a defense mechanism cells use to expose pathogens so immune cells can root them out and destroy them.
“By not killing the cells, the fungus can linger in the tissue and go into deeper layers of the skin,” says Isidoro-Ayza.
With this new knowledge in hand, the researchers are hopeful that treatments and a potential vaccine are closer to becoming reality.
These findings are just one product of a collaboration between Klein and Isidoro-Ayza and scientists at the U.S. Fish and Wildlife Service and U.S. Geological Survey’s National Wildlife Health Center in Madison. The effort received $2 million in funding from the National Science Foundation and Paul G. Allen Family Foundation in 2023 to search for better insight into how P. destructans causes infection and develop treatment and prevention strategies against white-nose syndrome.
The research is valuable not only for the conservation of bats, which provide a number of benefits including as pollinators and insect predators, but because fungal pathogens are a growing problem for many species.
“There are fungal diseases causing epidemics and pandemics in different types of organisms, including plants, invertebrate animals, amphibians, reptiles and bats,” says Isidoro-Ayza. So, any mechanism that we discover or better understand in this disease could have implications for the conservation of other species too.”
This research was supported by funding from The U.S. Geological Survey (G20AC00050), the U.S. Fish & Wildlife Service (13190304), the Canadian Institute for Advanced Research (MSN247980), the National Science Foundation (2301729), La Caixa Foundation fellowship (ID 100010434) under agreement LCF/BQ/AA17/11610020, National Institutes of Health (T32OD010423) and the Morris Animal Foundation (D23ZO-461).

