The known: The Victorian population was largely immunologically naïve to the Japanese encephalitis virus (JEV) before the 2022 outbreak. As most JEV infections are asymptomatic, their prevalence during the outbreak was unknown.
The new: We found evidence of JEV infections across northern Victoria, including in areas where cases of Japanese encephalitis had not been reported. Potential risk factors for infection were higher age and exposure to feral pigs.
The implications: It is uncertain whether JEV will become endemic in northern Victoria, but expanding vaccine eligibility may be required to protect residents against infection.
Japanese encephalitis virus (JEV) is a zoonotic mosquito‐transmitted flavivirus.1 Water birds maintain and amplify the virus, and pigs are important amplifying hosts for transmission to humans, primarily via Culex mosquitoes.2 Most JEV infections are asymptomatic, but 0.1–1% result in Japanese encephalitis, a life‐threatening illness.1,3 JEV causes substantial morbidity and mortality in the regions of South‐East Asia and the Western Pacific in which the virus is endemic.4 The case fatality rate for Japanese encephalitis is 20–30%,1,3 and 30–50% of people who have had Japanese encephalitis experience long term neurological sequelae.1,3,5
The first outbreak of JEV infections in southeast Australia was reported after detection of the virus in piggeries in February 2022 and the targeted investigation of cases of encephalitis in humans during March in Victoria, New South Wales, Queensland, and South Australia.6,7 The JEV outbreak was declared a communicable disease incident of national significance on 4 March 2022.8 In the initial outbreak, a total of 42 infections (32 confirmed, ten probable) were reported, including 13 in Victoria.8,9 As estimates of the ratio of clinical to subclinical cases of JEV infection range from 1 in 25 to 1 in 1000,2 thousands of JEV infections may have been undetected during the outbreak.6,10,11
As part of the initial public health response, specific population groups were prioritised for vaccination from March 2022.8,11 However, future prevention strategies, including a broader vaccination program, will require further characterisation of who is at greatest risk of JEV infection. We therefore investigated the distribution and prevalence of JEV antibody (as evidence of past infection) in northern Victoria following the 2022 outbreak, seeking to identify groups of people at particular risk of infection.
Two flaviviruses closely related to JEV, the Murray Valley encephalitis virus (MVEV) and the Kunjin subtype of the West Nile virus (KUNV), are endemic to Australia. Outbreaks of Murray Valley encephalitis were recorded in southern Australia in 1951, 1974, and 2011, but no human infections with MVEV were reported in Victoria during 2011.12 Following the detection of MVEV and KUNV in mosquitoes in Victoria in early 2023,13 we undertook additional retrospective testing of samples collected for our JEV antibody serosurvey to clarify interpretation of its results, to assess the seroprevalence of antibodies to MVEV and KUNV, and to inform public health actions.
Methods
We undertook our cross‐sectional serosurvey as part of a national JEV serosurveillance program.14 We report our study in accordance with the STROBE statement on cross‐sectional studies.15
Recruitment
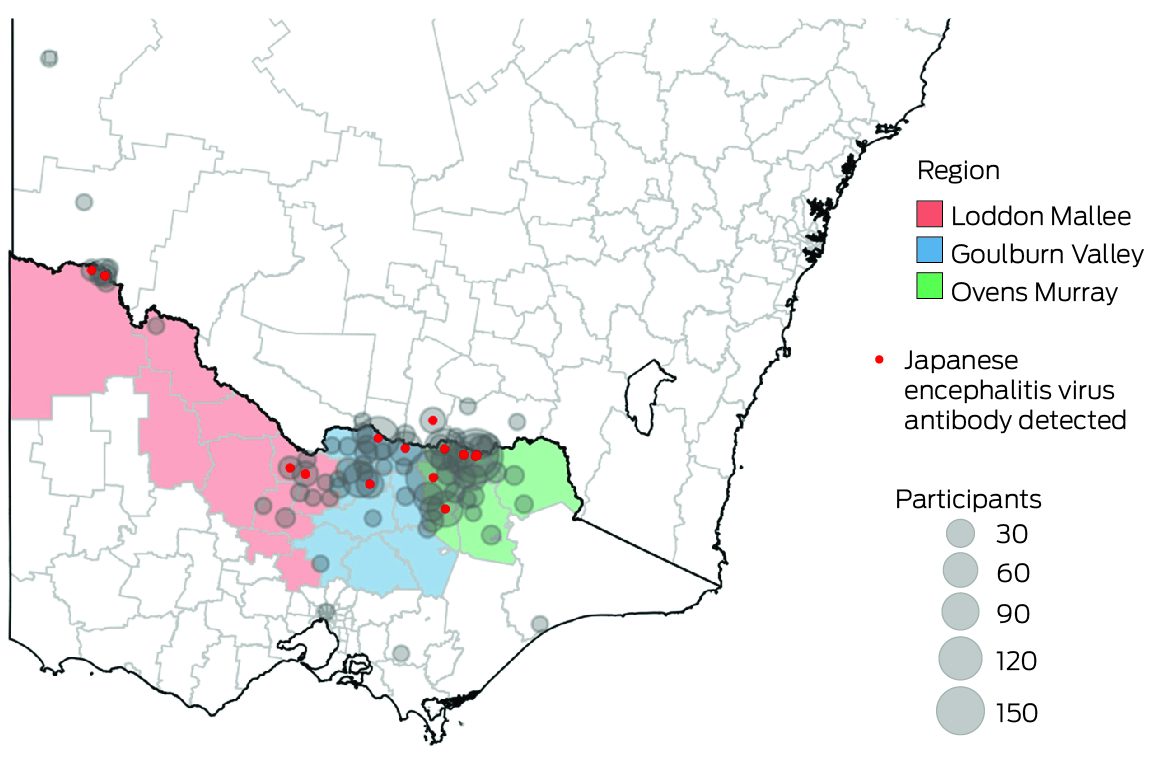
We recruited participants in the catchment areas of three Victorian local public health units (Ovens Murray, Goulburn Valley, Loddon Mallee; Box 1) during 8 August – 1 December 2022. People in these areas were deemed to be at high risk of JEV infection, based on the likely locations of exposure of people who had been diagnosed with Japanese encephalitis, the locations of piggeries with infected animals, and detections of JEV‐positive mosquitoes during the 2022 outbreak. We undertook both opportunistic recruitment of people from whom blood was collected at participating pathology centres for other purposes, as well as targeted recruitment of people less likely to be reached in this manner (eg, younger men) by community outreach (phone call or email, at meetings with community groups), and through radio, newspaper, and social media advertisements.
Participants provided informed consent in an online form; parent or guardian consent was also required for people under 18 years of age. Participants completed an online questionnaire about potential exposure and risk factors, and a blood sample was subsequently collected by phlebotomists at Dorevitch and Austin Pathology collection centres. Participants found to be seropositive for JEV antibody completed a follow‐up telephone questionnaire to confirm their responses to the online questionnaire and to provide additional information about risk factors and potential exposure locations.
People were ineligible for the study if they had previously been diagnosed with or vaccinated against Japanese encephalitis, or had been born in a country or territory where JEV is endemic (Bangladesh, Brunei, Burma, Cambodia, China, Guam, India, Indonesia, Japan, Laos, Malaysia, Nepal, North Korea, Pakistan, Papua New Guinea, the Philippines, Russia, Saipan, Singapore, South Korea, Sri Lanka, Taiwan, Thailand, Timor‐Leste, Vietnam).
Laboratory testing
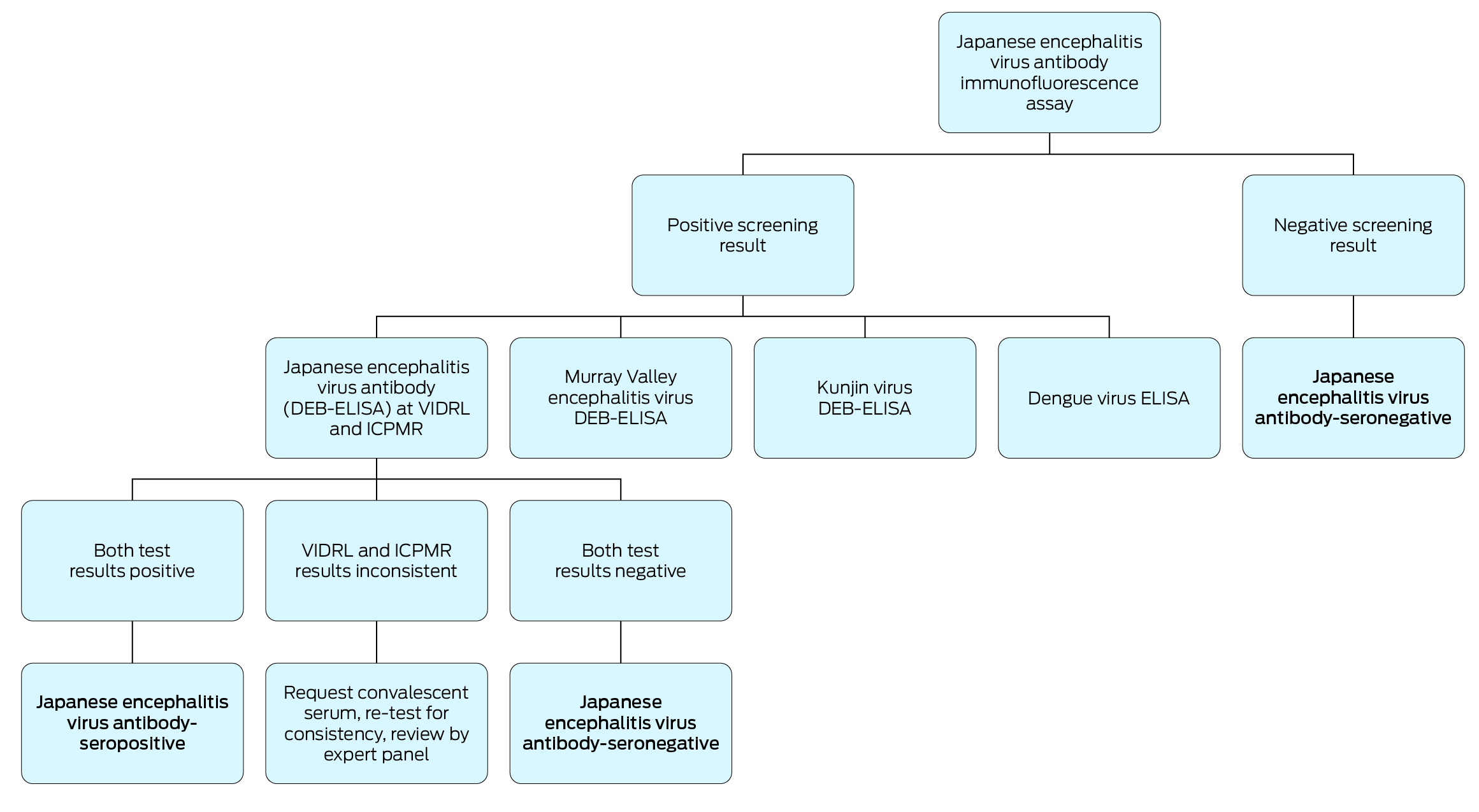
Participants were tested for JEV IgM and IgG antibodies at the Victorian Infectious Diseases Reference Laboratory (VIDRL), using the EUROIMMUN immunofluorescence assay for the JEV genotype I envelope protein (strain M28, taxonomy ID 2555554), performed in accordance with the manufacturer’s instructions.16,17 If the immunofluorescence test result was positive, testing for other flaviviruses was undertaken to assess cross‐reactivity (Dengue virus IgM and IgG, using an enzyme‐linked immunosorbent assay [ELISA]; MVEV total antibody, KUNV total antibody, and JEV total antibody using validated in‐house defined epitope‐blocking [DEB]‐ELISAs).18 Samples were also sent for parallel JEV total antibody testing with an in‐house DEB‐ELISA at the Institute of Clinical Pathology and Medical Research in Sydney. An expert review panel reviewed all serological results and questionnaire responses from all participants with positive immunofluorescence results; the panel classified participants as seropositive if evidence of prior JEV infection was found (JEV IgG‐positive and JEV total antibody‐positive) and as seronegative otherwise (JEV total antibody‐negative, or inconsistent results, suggesting a false positive result). The expert review panel could request an additional blood sample from individual participants for confirmatory testing, provided they had not since been vaccinated against JEV (Box 2).
If the residual sample volume from participants with negative immunofluorescence screening results was sufficient, it was tested in 2023 for MVEV and KUNV total antibody at the VIDRL using in‐house DEB‐ELISAs.
Statistical analysis
The primary outcome was seroprevalence of JEV IgG antibody, overall and by selected factors of interest (occupations, water body exposure, recreational activities and locations, exposure to animals, protective measures). Secondary outcomes were MVEV and KUNV antibody seroprevalence. We report descriptive statistics; the statistical significance of differences was assessed using Fisher exact tests (categorical variables) or by logistic regression (age). We estimated prevalence odds ratios (PORs) with 95% confidence intervals (CIs), assuming a binomial distribution. Statistical analyses were undertaken in Stata 16; maps were produced in R 4.1.2 (R Foundation for Statistical Computing).
Ethics approval
The Sydney Children’s Hospital Network Human Research Ethics Committee approved the study (2022/ETH01167).
Results
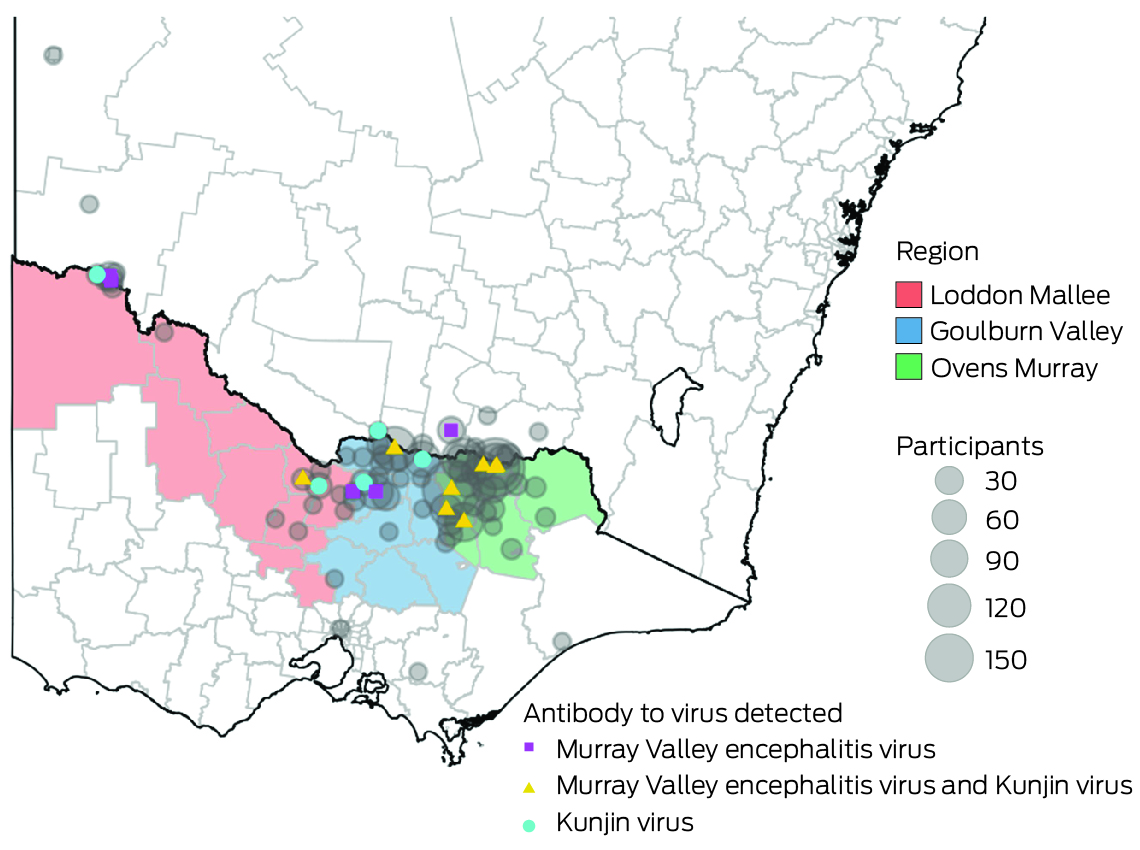
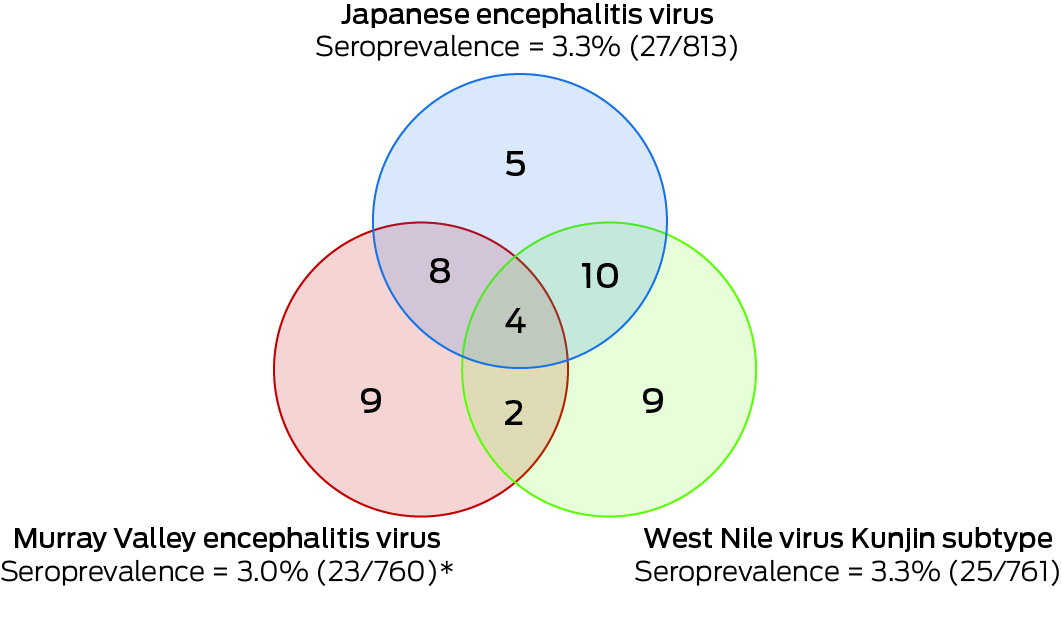
A total of 813 participants were recruited: 555 in the Ovens Murray region, 195 in Goulburn Valley, and 63 in Loddon Mallee. Twenty‐seven participants (3.3%; 95% CI, 2.2–4.8%) were JEV IgG‐positive, but none were IgM‐positive, suggesting past JEV infections, but not immediately before testing. Twenty of 660 opportunistic recruits (3.0%) and seven of 153 targeted recruits (4.6%) were JEV IgG‐seropositive. Eight of 27 JEV IgG‐seropositive participants were also seropositive for MVEV total antibody, ten for KUNV total antibody, and four for all three flaviviruses. Twenty‐three of the JEV IgG‐seropositive participants completed follow‐up questionnaires.
In total, 761 participants were tested for MVEV and KUNV total antibody. Nine were positive for MVEV total antibody, nine for KUNV total antibody, and two for both MVEV and KUNV total antibody. One sample had an equivocal result for MVEV total antibody and was excluded from subsequent analyses. Overall MVEV seroprevalence was 23 of 760 (3.0%; 95% CI, 1.9–4.5%), and KUNV seroprevalence 25 of 761 (3.3%; 95% CI, 2.1–4.8%) (Box 3).
Demographic factors
All 27 JEV IgG‐seropositive participants were born in Australia (Box 4), sixteen reported in the initial questionnaire never having travelled to a country in which JEV is endemic, and six (of 23 respondents) reported in the follow‐up questionnaire that they had spent more than one month in such a region. The median age of all participants was 59 years (interquartile range, 42–69 years); the median age of JEV‐seropositive participants was 73 years (interquartile range, 63–78 years). The estimated prevalence of infection increased with age for all flaviviruses: JEV IgG antibody: POR (per year), 1.07 (95% CI, 1.03–1.10); MVEV total antibody: POR, 1.04 (95% CI, 1.01–1.07); KUNV total antibody: POR, 1.07 (95% CI, 1.04–1.11).
The proportion of JEV IgG‐seropositive participants who were male (14 of 27, 52%) was larger than the male proportion of all participants (317 of 813, 39%), but differences by sex in the estimated prevalence of infection were not significant (overall: male participants, 4% [95% CI, 2–7%]; female participants, 3% [95% CI, 1–4%]; Box 5), nor were differences by sex for MVEV or KUNV antibody statistically significant (data not shown).
Geographic factors
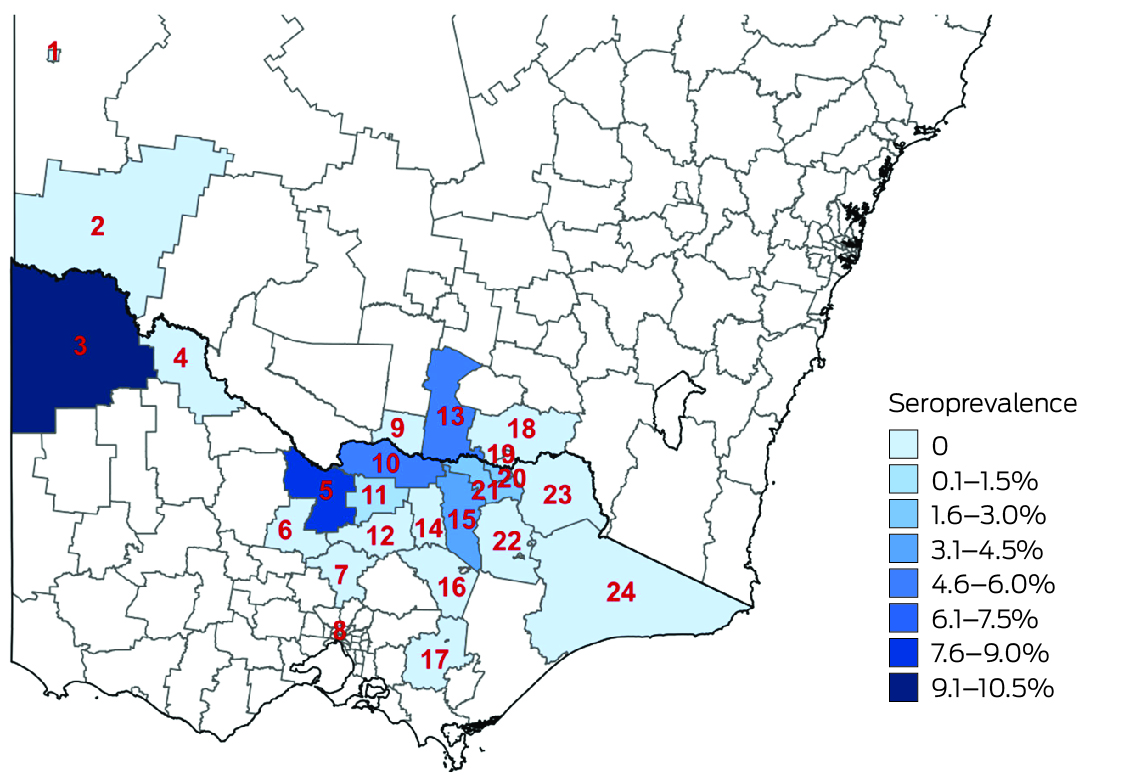
Most participants resided in the regions in which they were recruited (766 of 813, 94.2%) and near the primary recruitment locations (Box 6). Among these participants, JEV IgG seroprevalence was highest in Loddon Mallee (five of 59 participants, 8% [95% CI, 3–19%]) and lowest in Ovens Murray (14 of 518, 3% [95% CI, 1–4%]) (Box 7). Among all 813 serosurvey participants, JEV IgG seroprevalence was generally higher in northwest and mid‐north local government areas and lower in eastern and southern local government areas (Box 8). Most local government areas in which JEV IgG‐seropositive participants resided were near the Murray River (Box 6). There were no JEV IgG‐seropositive participants in 16 local government areas, all with low numbers of participants (Supporting Information, table 1). Antibodies to MVEV and KUNV were also detected in participants from across northern Victoria; MVEV and KUNV antibody seroprevalence were both highest in Loddon Mallee (Box 9).
Exposure factors
None of the associations of JEV IgG‐seropositivity with a broad range of investigated factors — occupation, water exposure, activities and locations, animal exposure, protective measures — were statistically significant, with the exception of exposure to feral pigs (POR, 21; 95% CI, 1.7–190) (Supporting Information, table 2). The five participants who reported this exposure (two JEV IgG‐seropositive, three seronegative) noted that contact was infrequent (a few times a year). JEV IgG‐seropositive participants more frequently reported some risk factors than did seronegative participants, such as daily exposure to mosquitoes and visiting farms at least a few times a month, but the differences were not statistically significant, nor did the amount of time spent outdoors for work or recreation or at dawn or dusk differ significantly between JEV IgG‐seropositive and ‐seronegative participants (data not shown). Similarly, most associations of MVEV or KUNV antibody seropositivity with reported exposure factors were not statistically significant, with two exceptions: exposure to water irrigation systems increased the risk of MVEV antibody seropositivity (POR, 3.8; 95% CI, 1.5–10.0), and using mosquito repellent when mosquitoes were about reduced it (POR, 0.30; 95% CI, 0.11–0.90) (Supporting Information, tables 3, 4).
Discussion
We report the first serosurvey to assess population exposure to JEV in Victoria. Similar to those of serosurveillance activities in NSW,19 our findings suggest that many more people were infected with JEV in northern Victoria during the 2022 outbreak than detected by case‐based reporting. We also found evidence for past infection with MVEV and KUNV in northern Victoria, and that a large proportion of people in this region are vulnerable to JEV, MVEV, and KUNV infections. These findings support the need for a JEV vaccination program and other prevention strategies targeting these flaviviruses to reduce the risk of human infections during future mosquito seasons. Vaccines for protecting against MVEV or KUNV are not yet available.
We found evidence of past JEV infections across northern Victoria, including locations where JEV had previously been detected only in animals. Official advice during the initial stages of the 2022 Japanese encephalitis outbreak emphasised the risk along the Murray River and the border with NSW, but we also found evidence of infections among people living in regional and rural towns further south. Our findings support the progressive expansion of JEV vaccine eligibility in Victoria during the outbreak — local government areas deemed to be at high risk were initially the priority, but further local government areas were included as vaccine supply increased — but also highlight the limitations of estimating risk on the basis of limited surveillance data. As the bird and mosquito species relevant to JEV transmission are widely distributed in Victoria, the geographic range of the virus could expand in future seasons.20,21 A broader surveillance program could better determine JEV distribution in Victoria and identify changes in geographic risk that indicate the need for a more extensive vaccination program.
Children are at greater risk of JEV infection than older people in countries where the virus is endemic, as most adults are exposed to infection during childhood.1,22,23 However, our findings and the preliminary results of the NSW serosurvey19 suggest that in immunologically naïve Australian populations, older adults were more likely to be JEV IgG‐seropositive. Potential explanations include age‐related predisposition to infection, differing exposures by age group (eg, occupational and recreational activities), or the greater lifetime risk of exposure through travel. Older seropositive participants may also have been exposed to other flaviviruses, such as MVEV, including during the large 1974 outbreak (25 of 27 JEV IgG‐seropositive participants were aged 50 years or more), and our test results may have been confounded by antigenic cross‐reactivity.24 Further, JEV may have circulated undetected in southeastern Australia prior to 2022, leading to acquired immunity in older people,25 but there is no further evidence supporting this possibility. In any case, our results suggest that older adults are at greater risk of JEV infection than younger people, supporting the decision, given limited vaccine supply, to restrict JEV vaccination in Victoria early in the 2022 outbreak to people aged 50 years or more. We found that sex was not a statistically significant risk factor for infection, but in regions where JEV is endemic boys and men are more likely to be infected, experience clinical illness, and have poor outcomes than girls and women.5,22 As 18 of 45 cases of Japanese encephalitis in Australia since 2021 were in men aged 60 years or older,26 further investigations in immunologically naïve populations are needed.
Other than age, the only factor associated with JEV IgG‐seropositivity in our study was contact with feral pigs, but only five participants reported such exposure, and described it as infrequent. Contact with domestic pigs was not associated with JEV IgG‐seropositivity, but many piggery workers were ineligible for our study because they had been vaccinated against JEV. The virus was detected in feral pigs in other Australian states and territories during the 2022 outbreak,7 but surveillance in Victoria was focused on domestic piggeries. Expanding surveillance programs to include feral pigs could better assess the risk associated with these animals.
JEV IgG seroprevalence in our study was lower than in neighbouring parts of NSW.19 The risk of infection may be influenced by geographic factors, but the difference could also be related to differences in testing methodology (we forwarded samples to the Institute of Clinical Pathology and Medical Research for testing only after a positive immunofluorescence screening result) or recruitment strategies (the NSW serosurvey relied solely on advertising and community outreach, and we found a slightly higher seroprevalence among participants recruited using targeted strategies compared to those recruited opportunistically). Results from serosurveys in other Australian states and territories, and incorporating all clinical case‐based data and animal and vector surveillance data, could shed further light on these differences in affected areas.
We found evidence of prior MVEV infections among participants aged 20–29 years, who could not have been infected during the 1974 outbreak. MVEV may have continued to circulate in Victoria without being detected in mosquitoes or humans, or these people could have been infected in other parts of Australia. Given these findings and the 2023 Murray Valley encephalitis outbreak,27 seropositivity for all flaviviruses and cross‐reactivity should be investigated. An MVEV serosurvey in high‐risk areas of regional Victoria is underway.28
Limitations
As we aimed to rapidly assess risk factors for JEV IgG seroprevalence in regional areas where cases of Japanese encephalitis had been reported, we did not include an urban control group. As 61% of our participants were female and 49% aged 60 years or older, our sample was probably not representative. Further, 81% of participants were recruited opportunistically; they may have had medical conditions that influenced their risk of JEV exposure. Participation was hampered in some areas by extensive flooding in northern Victoria during October and November 2022. Recruitment varied between locations, influenced by the capacity of the local pathology centre, the availability of staff, and opportunities for recruiting participants. This variation will have influenced the overall seroprevalence estimate, particularly as seroprevalence was highest in locations that provided the fewest participants. Some seropositive individuals may have been infected while traveling abroad; six JEV IgG‐seropositive participants reported having spent more than one month in regions where the virus is endemic.
We used several assays to determine seropositivity, but the close antigenic relationship between flaviviruses complicates the interpretation of serological results.29 Some participants were seropositive for antibodies to several flaviviruses, which could reflect either multiple infections or cross‐reactivity. The DEB‐ELISAs employed are highly specific because they use immunodominant epitopes on JEV, MVEV, and KUNV proteins and virus‐specific monoclonal antibodies.29 Nevertheless, misclassification of participants is possible. As JEV has rarely emerged in regions where MVEV is endemic, information about their cross‐reactivity is limited.10,30
Conclusion
Our findings regarding the pattern and extent of JEV infection in northern Victoria following the 2022 outbreak provides a foundation for further flavivirus research in this region and have implications for people in Australia and overseas where JEV could emerge.31 As appropriate hosts and mosquito vectors are present, JEV is likely to become established in year‐round transmission cycles in northern Australia, with occasional extension into temperate regions when environmental conditions are suitable.32 Our findings support recommendations regarding the use of limited vaccine supplies and indicate the need for alternative prevention strategies. As we identified population vulnerability to JEV infection but no clear risk factors for infection, further expanding vaccine eligibility criteria may be required to prevent infections.
Box 1 – Primary recruitment locations for the serosurvey of the prevalence of antibodies to Japanese encephalitis virus and other flaviviruses in three Victorian local public health unit catchment areas, 8 August – 1 December 2022
Box 2 – Testing algorithm for the serosurvey of the prevalence of antibodies to Japanese encephalitis virus and related flaviviruses in three Victorian local public health unit catchment areas, 8 August – 1 December 2022
Box 3 – Summary of flavivirus antibody seroprevalence in three Victorian local public health unit catchment areas, 8 August – 1 December 2022
Box 4 – Flavivirus antibody seroprevalence in three Victorian local public health unit catchment areas, 8 August – 1 December 2022, by participant characteristic
|
|
Primary outcome |
Secondary outcomes |
|||||||||||||
|
Characteristic |
Japanese encephalitis virus (JEV) |
Murray Valley encephalitis virus |
West Nile virus Kunjin subtype |
||||||||||||
|
|
|||||||||||||||
|
Total |
27/813 (3%) |
23/760* (3%) |
25/761 (3%) |
||||||||||||
|
Age (years) |
|
|
|
||||||||||||
|
0–9 |
0/1 |
0/1 |
0/1 |
||||||||||||
|
10–19 |
0/21 |
0/19 |
0/19 |
||||||||||||
|
20–29 |
1/64 (2%) |
3/62 (5%) |
0/62 |
||||||||||||
|
30–39 |
1/104 (1%) |
1/95 (1%) |
0/95 |
||||||||||||
|
40–49 |
0/90 |
0/88 |
0/88 |
||||||||||||
|
50–59 |
3/134 (2%) |
2/129* (2%) |
2/130 (2%) |
||||||||||||
|
60–69 |
5/202 (2%) |
3/184 (2%) |
9/184 (5%) |
||||||||||||
|
70–79 |
11/151 (7%) |
9/139 (6%) |
9/139 (6%) |
||||||||||||
|
80 or older |
6/46 (13%) |
5/43 (12%) |
5/43 (12%) |
||||||||||||
|
Sex |
|
|
|
||||||||||||
|
Male |
14/317 (4%) |
10/291 (3%) |
14/291 (5%) |
||||||||||||
|
Female |
13/496 (3%) |
13/469* (3%) |
11/470 (2%) |
||||||||||||
|
Indigenous status |
|
|
|
||||||||||||
|
Non‐Indigenous |
25/782 (3%) |
22/731* (3%) |
22/732 (3%) |
||||||||||||
|
Aboriginal |
1/13 (8%) |
1/13 (8%) |
2/13 (15%) |
||||||||||||
|
Torres Strait Islander |
0/1 |
0/1 |
0/1 |
||||||||||||
|
Prefer not to say |
1/17 (6%) |
0/15 |
1/15 (7%) |
||||||||||||
|
Country of birth |
|
|
|
||||||||||||
|
Australia |
27/740 (4%) |
22/691* (3%) |
25/692 (4%) |
||||||||||||
|
Other country in which JEV is not endemic |
0/73 |
1/69 (2%) |
0/69 |
||||||||||||
|
|
|||||||||||||||
|
* Excludes one sample for which the result was equivocal. |
|||||||||||||||
Box 5 – Seroprevalence of Japanese encephalitis virus antibody, by sex and age
|
Age (years) |
Male participants |
Female participants |
|||||||||||||
|
Number |
Proportion (95% CI) |
Number |
Proportion (95% CI) |
||||||||||||
|
|
|||||||||||||||
|
All ages |
14/317 |
4% (2–7%) |
13/496 |
3% (1–4%) |
|||||||||||
|
0–9 |
0/1 |
0 (0–98%) |
0 |
— |
|||||||||||
|
10–19 |
0/7 |
0 (0–41%) |
0/14 |
0 (0–23%) |
|||||||||||
|
20–29 |
0/11 |
0 (0–28%) |
1/53 |
2% (0–10%) |
|||||||||||
|
30–39 |
0/24 |
0 (0–14%) |
1/80 |
1% (0–7%) |
|||||||||||
|
40–49 |
0/35 |
0 (0–10%) |
0/55 |
0 (0–6%) |
|||||||||||
|
50–59 |
0/51 |
0 (0–7%) |
3/83 |
4% (1–10%) |
|||||||||||
|
60–69 |
3/86 |
3% (1–10%) |
2/116 |
2% (0–6%) |
|||||||||||
|
70–79 |
7/80 |
9% (4–17%) |
4/71 |
6% (2–14%) |
|||||||||||
|
80 or older |
4/22 |
18% (5–40%) |
2/24 |
8% (1–27%) |
|||||||||||
|
|
|||||||||||||||
|
CI = confidence interval. |
|||||||||||||||
Box 6 – Location of participants in the Japanese encephalitis virus antibody serosurvey and of seropositive participants (by residential postcode)
Box 7 – Japanese encephalitis virus IgG seroprevalence by Victorian local public health units, for the 766 participants living within the respective catchment areas
|
Region |
Number |
Proportion (95% CI) |
|||||||||||||
|
|
|||||||||||||||
|
All participants* |
26/766 |
3% (2–5%) |
|||||||||||||
|
Ovens Murray |
14/518 |
3% (1–4%) |
|||||||||||||
|
Goulburn Valley |
7/189 |
4% (2–7%) |
|||||||||||||
|
Loddon Mallee |
5/59 |
8% (3–19%) |
|||||||||||||
|
|
|||||||||||||||
|
* One seropositive participant living in a border community had a New South Wales address. |
|||||||||||||||