Papillomaviruses are compact, non-enveloped, icosahedral viruses with a diameter of 55 nm. They possess a double-stranded, circular DNA genome that is around 8000 base pairs in length [29]. . These tumors may be seen in a wide range of higher animals, including mammals and birds, affecting the skin and mucous membranes [30]. The tumors they induce vary in kind, ranging from non-cancerous growths such as warts, papillomas, and condylomas, to cancerous growths, primarily squamous cell carcinomas [31]. Due to the severe infections and illnesses they cause in the anogenital tract, a subset of approximately 40 of these viruses has become very important in public and medical health [2].
CC is caused by a persistent infection with one or more genotypes of oncogenic HPV, which is found in the majority of patients with cervical carcinoma [32, 33]. A comprehensive assemblage of over 200 distinct HPV genotypes has been identified and categorized according to their propensity to induce cancer as high-risk HPV (HR-HPV) or low-risk HPV (LR-HPV). Particularly prevalent in invasive CC on a global scale were the HPV genotypes 16, 18, 31, 33, 35, 45, 52, and 58. In the interim, it was documented that there were substantial variations in the distribution of HPV genotypes across countries, including within regions of the same nation [34].
Sexually transmitted diseases can be transmitted through direct sexual contact, including vaginal, anal, and oral intercourse, from over 40 different varieties of HPV [35, 36]. HPVs cause a variety of malignancies by replicating in stratified squamous epithelia. The present scientific endeavors in the field of HPV biology are centered on comprehending the dynamics between the virus and the host, which facilitate HPV’s ability to endure in the tissue for years or even decades [37].
HPVs are classified into five genera based on changes in the sequence of the L1 open reading frame (ORF), which contains the genetic information for the main capsid protein. The types of papillomaviruses may be categorized into several groups: Alpha-HPV, Beta-HPV, Gamma-HPV, Mupapillomavirus (mu-HPV), and Nupapillomavirus (nu-HPV). While most of these viruses belong to the Gamma-HPV, Alpha-HPV, and Beta-HPV groups [38]. A further distinction made historically between HPVs has been made regarding their affinity for mucosal or cutaneous epithelia. Therefore, in the literature, Beta- and Gamma-HPVs are characterized as “cutaneous,” whereas Alpha-HPVs are still referred to as “mucosal.” Nonetheless, mounting data indicates that “cutaneous” HPV is widely distributed at mucosal locations [39,40,41]. Alpha-HPVs are categorized into two groups: HR and LR, according to the International Agency for Research on Cancer (IARC) carcinogenic potential [42]. The HR types of HPV are unique in their oncogenic capacity due to their role as causative agents of head and neck squamous cell carcinoma (HNSCC), vulvar, penile, and vulvar cancer. More specifically, the development of oropharyngeal squamous cell carcinoma (OPSCC) is mainly attributable to HPV [43]. The percentage of cases in which HPV is responsible for the neoplasms mentioned above varies from 25% (vulvar carcinoma) to almost 100% (cervical and anal carcinomas). In particular, HPV-16 is the most prevalent form among all malignancies associated with HPV, accounting for 60–90% of cases in conjunction with HPV-18 [18]. Beta-HPVs are found in the hair follicles of the genital epidermis, forehead, back of the hand, buttocks, and buttocks, which constitute their natural reservoir. However, as previously stated, the dissemination of Beta and Gamma HPVs also encompasses anatomical sites outside the skin, including the nasal mucosa, oral cavity, and anal canal [44].
The genomes of HPVs are circular, about 7.9 kb double-stranded DNA, with eight major expressed protein-coding ORFs, an intergenic noncoding region (NCR) containing simple (AT)n and poly-T repeats, and an upstream regulatory region (URR). The ORFs are designated E6, E7, E1, E2, E4, E5, L2, and L1 (listed 5′–3′) based on the estimated time of their expression throughout the viral life cycle, where “E” indicates early and “L” indicates late. At certain phases of infection, E8—a sequence often 12 2/3 codons in length—is spliced to E2 to generate E8^E2, in addition to the significant ORFs. All ORFs are expressed as polycistronic (multi-ORF) mRNAs and are located on the sense (forward) strand [45, 46]. Two polyadenylations (pA) signals, viral early (pAE) and viral late (pAL), can be used to divide the genomes of all papillomaviruses into three distinct regions: an URR, an early (E), and a late (L) gene region. Since HPV does not produce its RNA polymerase, it relies on the transcription of the host RNA polymerase II. Therefore, the functionality of viral promoters is entirely governed by chromatin modifications and transcription factors of the host and virus. Using host polyadenylation machinery, the viral transcripts originating from each promoter are subsequently polyadenylated, either at an early pAE or a late pAL site. pAE and HPV early promoters become active during the early phase of the viral life cycle, while pAL and viral late promoters become active during the late phase [46, 47]. More precisely, the incorporation of E6/E7 genes from the HPV genome into the genetic material of the host, followed by the expression of these genes by the host, maintains an altered phenotype characteristic that has been linked to the formation of cancer [48]. HPV can attack mucous membranes and change the cells in the cervix. This is caused by a sexually transmitted disease [49]. The oncogenes E6 and E7 are essential to HPV infection. Finding these genes to identify HPV strains, especially the HPV-16 strain, would have a substantial effect because of its outstanding sensitivity ; the dielectric electrochemical biosensor stands out among other pathogen detection techniques [50]. New data shows that E6 and E7 are also crucial for stopping the host cell’s natural defensive response to HPV [51]. Together with other biological cues, the E1 and E2 proteins drive viral propagation. Additionally, E2 has been connected to the control of transcription in cells and viruses. The purpose of other viral proteins is still unknown after decades of study [52] (Fig. 1).
The genetic makeup and organization of HPV. An illustration of the genetic map of HPV-16 is shown. Open reading frames (ORFs) are denoted by solid bars. The six initial ORFs (E1, E2, E4, E5, E6, and E7) are expressed at distinct phases of epithelial development. The L1 and L2 ORFs are actively transcribed in cells undergoing viral DNA replication in highly differentiated epithelial cells [53]. Three sense-strand trinucleotide (codon) reading frames represent the approximately 7.9 kb circular double-stranded DNA genome of HPV16. Rectangles of color in the appropriate reading frame represent protein-coding ORFs. The three primary parts of the HPV genome are the long control region (LCR), the early and late regions, and the timing of viral protein production after viral entrance into the host cell. The early section primarily encodes regulatory proteins that are necessary for the transcription and replication of the virus as well as cell cycle regulation, which supports HPV’s ability to transform and become immortal. The two viral structural proteins, L1 and L2, required for capsid formation, are encoded in the late region. The majority of the regulatory DNA sequences, including the origin of DNA replication and enhancer and promoter regions, that are required for both viral gene expression and genome replication are found in the LCR [54]. E8 is completely encoded within E1, while E4 is completely encoded within E2. The E4 protein is classified as a regulatory protein, which is produced by the genes of early viruses. E1, E2, E4, E5, E6, and E7 proteins are responsible for viral genome replication and maintenance in infected cells. The designations for early and late promoters (p) are p97 and p670, whereas the designations for early and late polyadenylation sites (polyA) are polyAE and polyAL, respectively [45, 55]
HPV vaccination methods
HPV vaccines are some of the best vaccines out there. They were the first to stop infection by a sexually transmitted virus that affects the mucosa without specifically boosting mucosal immunity [56]. Currently, the Chinese market offers five different HPV vaccine types for private purchase: Cervarix® (imported HPV-2), Gardasil® (HPV-4), Gardasil®9 (HPV-9), Cecolin® (domestic HPV-2), and a recently developed bivalent vaccine made domestically called Waldrinvax™, Recombinant HPV Bivalent (Types 16, 18) [57].
In 2006, the first HPV vaccine got its stamp of approval. All vaccines are made to protect against HPV-16 and HPV-18, which are the kinds that cause most cancers linked to HPV. It was 2020, and HPV shots were now part of government vaccination programs in over 100 countries [58]. HPV infection may be prevented by vaccination, and therapeutic vaccination offers the chance to develop cellular immunity against pre-existing HPV infections and lesions as well as stop the spread of malignancy [59]. The HPV vaccines currently on the market are based on virus-like particles (VLPs) that naturally come together from the L1 major capsid protein [60]. The HPV vaccines currently on the market (Gardasil, Gardasil 9, and Cervarix) are meant to protect against HPV and are made from L1-based VLPs that have been added to other substances [61]. The nonavalent vaccination Gardasil 9® was approved in 2014 and targeted the following HPV strains: HPV-6, HPV-11, HPV-16, HPV-18, HPV-31, HPV-33, HPV-45, HPV-52, and HPV-58 [62, 63]. These three well-established vaccines use eukaryotic producer cells [64].
Nonetheless, there is still a market for HPV vaccine development, and Cervavac, a quadrivalent vaccination, was just introduced in India [65]. Based on VLPs, a recombinant, noninfectious assembly of the L1 HPV capsid protein, the vaccines work similarly. Because VLPs and infection-causing HPV virions have the same antigens, exposure to VLPs triggers a potent neutralizing antibody response that prevents HPV from being absorbed by the cervix’s basal epithelial cells. The vaccine’s effectiveness is due to this humoral reaction [66].
A worldwide double-blind experiment with women aged 16 to 25 evaluated the effectiveness of the nonavalent Gardasil® 9 vaccination. More than 14,000 volunteers were recruited, and in contrast to the previous studies, the quadrivalent Gardasil® HPV vaccination was administered to the control group. The effectiveness of the vaccination against high-grade cervical illness linked to HPV-31, HPV-33, HPV-45, HPV-52, and HPV-58 (i.e., HPV subtypes not protected by the quadrivalent vaccine) was 97.1%; the nonavalent group had 0.5 cases per 10,000 person-years, while the quadrivalent group had 18.1 instances. The two participant groups had similar abnormalities related to HPV-6, HPV-11, HPV-16, and HPV-18 (i.e., HPV subtypes covered by the quadrivalent vaccination). Additionally, the nonavalent vaccination exhibited antibody response for up to five years, and its immunogenicity against HPV-6, HPV-11, HPV-16, and HPV-18 was similar to that of the quadrivalent vaccine. The nonavalent vaccination, according to the scientists, may be able to prevent more incidences of CC by offering more extensive coverage and sustained high effectiveness against all vaccine HPV subtypes [66,67,68].
The VLPs cause large titers of virion-neutralizing antibodies and resemble real viruses visually. GSK and Merck & Co. were the two firms that started the first commercial development of HPV vaccinations. GSK created Cervarix, a bivalent vaccine comprising HPV-16 and HPV-18 VLPs. Merck created the first quadrivalent vaccination, Gardasil®, and it was approved in 2006. It targets HPV-6, HPV-11, HPV-16, and HPV-18 and protects against genital warts (GW), which HPV-6 and HPV-11 mostly cause [69].
The adjuvants and the producer cells for the viral L1 proteins are further distinctions between the two vaccinations. The proteins for Cervarix and Gardasil are made in yeast (Saccharomyces cerevisiae) and L1-recombinant baculovirus-infected insect cells, respectively [70]. Cervarix®, a bivalent vaccination that targets HPV-16 and HPV-18, was approved in 2007. While Gardasil includes an aluminum salt adjuvant (aluminum hydroxyphosphate sulfate), Cervarix features a patented adjuvant called AS04, which is made up of aluminum hydroxide with 3-deacylated mono-phosphoryl lipid A, a detoxified form of lipopolysaccharide, and a Toll-like receptor four agonists. Later, Merck created Gardasil 9, a nonavalent vaccination that is comparable to Gardasil but contains L1 VLPs of five additional carcinogenic types: HPV-31, 33, 45, 52, and 58. As a result, Gardasil 9 may provide type-specific protection against 90% of CC cases globally. More recently, producers in China, India, and other nations have begun developing L1 VLP HPV vaccines [71]. The WHO is now reviewing Cecolin, a bivalent vaccine produced by Xiamen Innovax Biotech and contains both HPV-16 and HPV-18 VLPs. The vaccine was approved in China in 2020. Escherichia coli (E. coli) produces the L1 proteins, and the vaccine contains an alum adjuvant [72].
In many Chinese locations, the bivalent Cecolin® vaccination effectiveness study included more than 7,000 female volunteers aged 18 and 45 in 2012 and 2013. The hepatitis E vaccination was given to the control group. 100% and 97.3%, respectively, of the vaccines were effective against high-grade genital illness and chronic infection linked to HPV-16 or HPV-18. To enable a subgroup analysis, the participants were age-stratified into two groups: 27–45 and 18–26. The Cecolin® vaccine is undergoing a phase 3 clinical study in Ghana and Bangladesh; findings are anticipated in 2023 (NCT04508309) [66, 73].
These and five other HR-HPV strains (31/33/45/52/58), which together account for around 90% of cervical and other HPV-related malignancies, are protected against by the nine-valent HPV (9vHPV) vaccination. 90% of instances of HPV-related GW are caused by HPV-6 and 11, which are also protected against by the 4vHPV and 9vHPV vaccinations. To prevent specific HPV-related illnesses and malignancies, HPV vaccines were initially approved in 2006 (4vHPV), 2007 (2vHPV), and 2014 (9vHPV) after their effectiveness, immunogenicity, and safety were established in clinical studies. Adults and adolescents who are older are also being considered for catch-up vaccinations. In the European Union, the 9vHPV vaccination is recommended for persons nine years and older, with no maximum age restriction. It protects against precancerous lesions and malignancies of the cervix, vulva, vagina, and anus caused by particular forms of HPV, as well as GW produced by a specific HPV type. The 9vHPV vaccine is authorized for use in females aged 9–45 in the US to prevent cervical, vulvar, vaginal, and anal (pre)cancers, as well as oropharyngeal and other head and neck cancers and GW. It is also approved for males aged 9–45 to prevent anal (pre)cancer, oropharyngeal and other head and neck cancers, and GW. Adults may benefit from HPV vaccinations as well, even though young teenagers are the main target population for immunization. Regardless of an adult’s HPV infection status, the US Advisory Committee on Immunization Practices advises regular catch-up immunization through age 26 and shared clinical decision-making about HPV vaccination until age 45 [74].
The countrywide implementation of HPV vaccination often encounters challenges, such as poor compliance with administering the second or third dosage and insufficient funding to provide the total doses of the HPV vaccine, particularly in low- and middle-income countries. Administering a single dose of an HPV vaccine has the potential to address these concerns and substantially mitigate these challenges effectively. Before making a choice, it is crucial to have reliable and measurable information about the effectiveness of a single-dose HPV vaccine. A systematic review compares the efficacy of a single dose of the HPV vaccine (one dose) to that of two and three doses regarding infection prevention and pre-cancer incidence. The majority of the studies included in this systematic review and meta-analysis provide evidence that a single dose of an HPV vaccine can generate HPV-specific antibodies for a duration of up to 8 years. This establishes immunogenic memory and demonstrates that a single dose is equally effective in preventing infection and pre-cancerous conditions as receiving two or more vaccine doses. Nevertheless, there is limited research elucidating why antibodies do not respond similarly to two or three dosages, and neither the incidence of pre-cancer nor the infection rate is significantly reduced. Consequently, further research and longer durations of study are necessary to establish unequivocally the efficacy of the one-dose HPV vaccine [57] (Table 1).
mRNA vaccine engineering
The differences in immunological responses to these various vaccines have been the subject of numerous studies, particularly comparing the reactions to the bivalent and quadrivalent vaccines. Considerable research has been devoted to examining antibody responses, given their critical role in mediating protection against infections. Although antibody concentrations diminish and memory immunity is formed and maintained through the presence of specific memory B and T cells, cell-mediated immunity to HPV antigens remains a crucial component in the fight against HPV infection. In addition to innate cells that participate in antigen presentation and early defense, cellular immunity also encompasses adaptive responses in which T helper cells facilitate the production and (re)activation of B cells, thus facilitating the production of high levels of antibodies. It has been demonstrated that all three vaccines elicit robust antibody responses against the different vaccine types; however, the bivalent vaccine elicited more significant quantities of HPV16/18-specific serum antibodies and more robust B-cell responses. This was primarily attributed to the AS04 adjuvant, which is believed to stimulate a more favorable Th1 response [84]. Initial and subsequent investigations evaluating the immunogenicity of the HPV 16/18 AS04-adjuvant vaccine in women aged 15 to 25 revealed that total IgG antibodies against HPV16 and anti-HPV18 peaked at month 7, reached a plateau between months 18 and 24 and remained stable for up to 76 months following vaccination. High concentrations of functional antibodies were further validated by using pseudovirion-based neutralization assays to measure the neutralizing antibodies. Then, 10 years after the initial vaccination, an assessment of the long-term immunogenicity of the HPV16/18 vaccine in the serum of females aged 15 to 55 revealed that anti-HPV16 seropositivity remained elevated in all age groups. Seropositive females for anti-HPV18 were more prevalent among those aged 15 to 25 (99.2%) compared to those aged 26 to 45 (93.7%) and 46 to 55 (83.8%) [85, 86]. Although the three approved vaccines exhibited comparable effectiveness against HPV infection and precancerous lesions in clinical trials, they diverged in immunogenicity, as confirmed by an array of assays. In head-to-head trials comparing three doses of Cervarix® and Gardasil® in females aged 18–45 and females aged 12–15, HPV-16 antibody levels were substantially lower in females aged 12–15 treated with Gardasil® compared to Cervarix®, although their patterns of peak and decline over time were comparable. In comparison to Cervarix®, HPV-18 antibody levels and seropositivity were notably diminished in Gardasil®. Additionally, memory B cell responses were diminished, specifically for HPV-18, and HPV-16 and HPV-18 specific CD4 + T cell responses were reduced by Gardasil® for up to 24 months following vaccination [87,88,89]. The goal of HPV prophylactic vaccinations is to prevent HPV infection by stimulating the body’s humoral immunity to produce neutralizing antibodies. Nevertheless, an organism that has already acquired the virus cannot be effectively treated with the HPV prophylactic vaccination. Furthermore, several early genes (E1, E2, E4, E5) and late genes (L1, L2) have been lost as a result of the integration of the viral genome into the host genome, rendering preventative vaccinations useless against HPV-related precancerous lesions and malignancies [28].
As of right now, the most extensively used vaccination is the mRNA vaccine, which has shown to be an auspicious treatment approach in immunotherapy. Malone et al. showed in 1989 that encapsulating a cationic lipid (N-(1-(2,3-dioleyloxy)propyl)-N, N, N-trimethylammonium chloride (DOTMA)) could efficiently transfect and express mRNA in a variety of eukaryotic cells [90]. Following this, complete expression of mRNA transcribed in vitro was observed in mouse skeletal muscle cells in 1990. This event marked the initial instance of practical in vitro mRNA expression, which validated the viability of mRNA vaccine development [91]. Prioritizing the development of DNA-based methods was necessary because of RNA’s instability, ineffective in vivo delivery, and tendency to trigger exaggerated inflammatory responses until the late 2000s. Vaccines targeting mRNA are a relatively new vaccine type that has a lot of potential [92]. A coding sequence, a 3’ poly A tail, a 5’cap structure, and 5’ and 3’ untranslated regions (UTR) structures make up the mRNA structure [16].
Numerous studies have demonstrated that mRNA is unintegrated (safe) and that the capacity for autonomous replication is exceptionally high in the new generation of self-amplifying mRNA vaccines (saRNA vaccines). The significant factors contributing to the comparatively sluggish advancement of mRNA vaccines are their inadequate stability and delivery efficiency in contrast to DNA vaccines. As a result, delivery vectors, such as DC vectors, protamine, cationic lipid delivery systems, and polymer materials, are frequently used to transport mRNA into the body [93]. mRNA technology is a desirable substitute for conventional or even DNA vaccines since it offers several benefits. mRNA is precise because it will only express a particular antigen and trigger a tailored immune response, unlike attenuated or inactivated vaccines [94].
Additionally, it induces the innate immune system and stimulates humoral and cellular immune responses. mRNA vaccines are safer and more effective than DNA-based vaccines since expression does not necessitate nuclear entry; the likelihood of random genome integration is virtually non-existent [16, 95]. Additionally, since mRNA is rapidly broken down by cellular processes and disappears within two to three days, expression of the coded antigens is only temporary [96]. The manufacturing process benefits from the adaptable nature of the mRNA vaccine platform as well; modifications to the encoded antigen do not impact the physical-chemical properties of the mRNA backbone, enabling standardized production. Furthermore, the utilization of an in vitro cell-free transcription reaction for production mitigates safety apprehensions associated with the potential presence of viral contaminants and cell-derived impurities that are frequently encountered on alternative platforms [97].
Because the mRNA is prone to degradation, stabilizing it might increase expression. The expression and stability of mRNA vaccines are influenced by several variables. The stability and translation efficiency of mRNA is directly influenced by its structural features, including the CAP, poly(A) tail, and UTRs. For mRNA to remain stable in the cytosol, the poly(A) tail and cap, found at the 5′ and 3′ ends of mRNA, are essential [98]. To circumvent this, mRNA can be delivered via dendritic cell transfection, conjugation with polymers, peptides, or lipid-based carriers, injection of bare mRNA, or conjugating mRNA with lipid-based carriers [99]. The four material types—polymers, peptides, protamines, and lipids—as well as their corresponding delivery methods will be covered by the mRNA vaccine delivery systems. This will improve the stability, effectiveness, and safety of the naked mRNA once it is administered [100,101,102].
Moreover, therapies and vaccines based on mRNA are produced via rapid, cell-free IVT. Synthetic mRNA is generated by transcribed in vitro by an RNA polymerase from a plasmid DNA (pDNA) template that has been prepared and linearized. The resulting mRNA typically consists of a 5′ cap and a template-encoded 3’ poly(A) tail. Additionally, antisense and double-stranded RNAs, which can trigger an innate immune response that inhibits cellular translation and causes adverse effects, must be eliminated from the mRNA [103]. Endosomal or cytosolic receptors within the cells recognize RNA, which may activate the type I interferon (IFN-I) pathway and promote the production of proinflammatory cytokines and chemokines. These signaling molecules cause the activation of antigen-presenting cells (APCs), which in turn triggers a potent adaptive response [104].
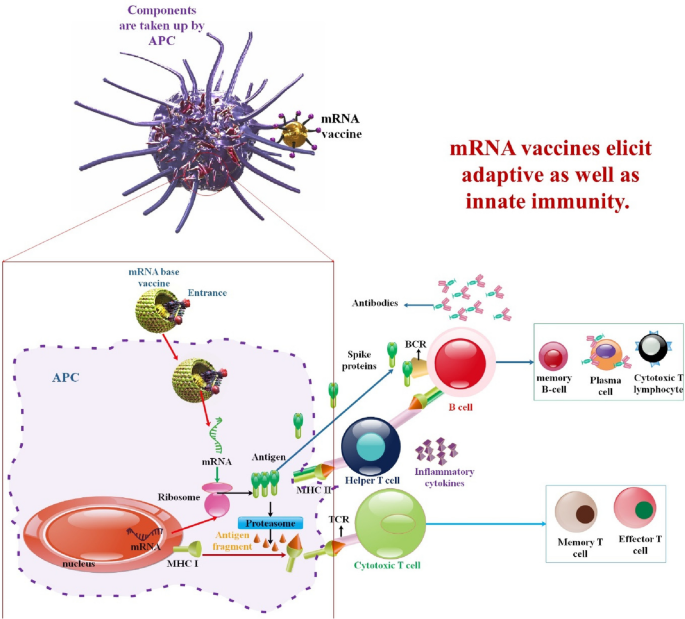
mRNA vaccines, instead of peptides, do not impose restrictions on MHC haplotypes. Moreover, since mRNA bonds to pattern recognition receptors, mRNA vaccines have the potential to function as self-adjuvants, a characteristic that is absent in vaccines composed of peptides and proteins. mRNA vaccines combine the untapped versatility of genetic vaccines with exceptional safety and desirable immunological properties [105]. mRNA vaccines are not limited by MHC haplotype and may elicit a balanced immune response that includes humoral and cellular immunity based on in situ protein production. Furthermore, since mRNA is a minimum and only transitory information carrier that does not interact with the genome, it is an essentially safe vector. mRNA vaccines also provide the more significant degree of development flexibility possible, as any protein may be created from mRNA without modifying the manufacturing procedure. When combined, mRNA offers a promising vector that might serve as the cornerstone of a revolutionary vaccine technology platform [106] (Fig. 2).
mRNA vaccines have dual impacts on immune activation. mRNA vaccines elicit adaptive as well as innate immunity. Antigen-presenting cells detect endocytosis of exogenous mRNA by TLR3 and TLR7/8 in the endosomes and RIG-1, NOD2, LGP2, and MDA-5 in the cytosol. This triggers robust IFN-I responses, which stimulate the production of proinflammatory cytokines and ultimately activate innate immunity (left). To activate CD4 + and CD8 + T cells, which aid in triggering adaptive immunity, peptides transcribed by re-endocytosed mRNA are displayed on MHC-I or MHC-II molecules (right). The release of proteins encoded by mRNA activates B cells [20, 107]
mRNA vaccines in HPV
mRNA-based therapeutics have enormous potential as clinical remedies; nevertheless, developing methods for delivering the bioactive agents in a safe, efficient, and immune-suppressant manner will be a significant obstacle to the realization of this class of medications [108, 109]. In the context of mRNA vaccines, scientists have made structural modifications to mRNA to improve its stability and facilitate systemic tolerance to antigenic presentation outside of inflammatory environments [110]. One significant challenge to treating HPV infections in the lower genital tract is the development of therapeutic vaccines. The development of therapeutic vaccines certainly seems like a good idea for medical care. Nevertheless, there aren’t any of these vaccines available for clinical use right now [111]. Therapeutic vaccines, in contrast to preventive vaccines, are designed to stimulate an immune response mediated by specific T cells. These devices are purposefully engineered to eradicate any remaining tumor lesions that may remain after an HPV infection, such as condyloma acuminata, a type of cervical atypical hyperplasia [109, 112]. Cervical intraepithelial lesions fall into two categories, as per the 5th edition of the WHO Classification of Female Genital Tumors: high-grade squamous intraepithelial lesions (HSIL), which includes cervical intraepithelial neoplasia grade 2 (CIN2) and CIN3 and low-grade squamous intraepithelial lesions (LSIL), which provides for CIN1. According to research data, after a 10-year follow-up period, 20–30% of HSIL cases may develop into invasive CC [113,114,115]. Currently, available treatments for LISL include lesions being removed and monitoring. With a 60% natural regression rate, LSIL has a high lesion persistence rate of 30% and a 10% progression rate to HSIL. Another meta-analysis revealed that 21% of LSIL will proceed to HSIL, 0.15% of LSIL will progress to an invasive cervical malignancy, and 48% of LSIL cleared spontaneously [116, 117]. Live vector vaccines are composed of viral or bacterial vectors. Listeria monocytogenes, Lactobacillus casei, and Lactococcus lactis are frequently utilized bacterial vector-based vaccines. The recombinant L. casei vaccine GLBL101c, which expresses HPV-16 E7, showed an E7-specific immune response and downgraded CIN3 lesion in a phase IIB study. The effectiveness of the alphavirus, adenovirus, and bovine pox virus has also been studied in clinical studies. However, the possible pathogenicity of the vectors and the existence of neutralizing antibodies, which restricts recurrent treatment, provide difficulties for using live vector vaccines in immunocompromised individuals [109, 112].
As determined by researchers, persistent HPV infection is a leading cause of cancer-related mortality in women, and there is an urgent need for the development of safe and effective therapeutic strategies. A comparison was made between three distinct mRNA vaccine modalities to assess their efficacy in targeting tumors that are linked to HPV-16 infection in mice. Self-amplifying mRNA encapsulated in lipid nanoparticles (LNPs), unmodified and nucleoside-modified non-replicating mRNA vaccines containing a chimeric protein formed by the fusion of the herpes simplex virus type 1 glycoprotein D (gDE7) and the HPV-16 E7 oncoprotein, were all produced by the researchers. It was shown that a single low-dose vaccination with any of the three gDE7 mRNA vaccines activated CD8 + T cells specific to E7, produced memory T cell responses that could stop tumor relapses, and eliminated subcutaneous tumors at various stages of development. Furthermore, after the administration of a single vaccination dosage, the gDE7 mRNA-LNP vaccines produced strong tumor protection in two distinct orthotopic mouse tumor models. Finally, comparison experiments showed that gDE7 mRNA-LNP vaccines, all three of them, outperformed gDE7 DNA and gDE7 recombinant protein vaccines [118].
Further examination reveals that while mRNA vaccines are acknowledged for their capacity to provoke immune responses targeted against infectious diseases, their impact on the functional commitment of CD8 + T cells in the tumor microenvironment (TME) and secondary lymphoid organs is still not well comprehended. This lack of understanding limits their potential for broader application in cancer immunotherapy. An investigation aimed to thoroughly assess the immune effects of an HPV E7 protein (HPV mRNA-LNP), a tumor-specific antigen of HPV-positive OPSCC, encoded in an LNP-encapsulated mRNA vaccine. HPV mRNA-LNP vaccination induced different patterns of immune response and fatigue in the spleen and TME. This vaccination had varying effects on the functional specialization of CD8 + T cells and activated both HPV-specific and total CD8 + T cells. The concurrent administration of immune checkpoint blockades and HPV mRNA-LNP vaccination increased HPV-specific CD8 + T cells while preserving their antitumor capabilities, thereby facilitating additional tumor regression. The combination of HPV mRNA-LNP vaccination and immune checkpoint blockade is a promising immunotherapeutic strategy for HPV-positive OPSCC, according to the findings of the researchers [119].
As they modulate cell cycle regulation, the E6 and E7 proteins of particular subtypes of HPV, including HPV-16 and 18, are strongly associated with CC, according to another study. The objective of this research endeavor was to examine the potential antitumor properties of a therapeutic vaccine (mHTV) composed of mRNA-HPV and non-oncogenic E6 and E7 proteins. To accomplish this, the vaccine was administered subcutaneously and intramuscularly to C57BL/6j mice, and the subsequent effects were assessed. In both subcutaneous and orthotopic tumor-implanted mice models, mHTV vaccination dramatically enhanced T cell-mediated immune responses and considerably inhibited tumor development, with a notable infiltration of immune cells into tumor tissues. In all mHTV-treated animals, tumor retransplantation at day 62 postprimary immunization stopped the tumor’s development. In addition, TC-1 transplantation substantially inhibited tumor growth 160 days after the most recent vaccination. mHTV-induced immunization of rhesus primates produced encouraging immune responses. The immunogenicity of mHTV in nonhuman primates provides solid support for its potential clinical application in humans to combat malignancies associated with HPV. Based on all available data, mHTV exhibits potential as a prophylactic and therapeutic vaccine [120].
For several reasons, the carcinogenic early proteins E6 and E7 are the most often targeted antigens by the HPV16 therapeutic vaccines now under development. (1) Since precancerous and cancerous lesions are the only places where E6 and E7 are expressed in large quantities, there is no significant risk that they will target healthy tissues; (2) Since E6 and E7 are necessary for the transformation and maintenance of infected cells, there is no significant risk that antigen loss-mediated immune escape will occur; 4) The immune response to E6 and E7 has been described preclinically and clinically; (3) no mechanisms of central tolerance against E6 and E7 have been revealed. Currently, the US FDA has not authorized any therapeutic HPV vaccination. Here, scientists present an mRNA-based vaccination that encodes for altered forms of the HPV16 early proteins E6 and E7. The vaccine is prepared as a lipid nanoparticle (LNP) based on MC3, and it may be injected intramuscularly. According to in vivo and in vitro research, the translated mRNA is functional and elicits a distinct adaptive immune response to the antigen. Vaccinated mice harboring HPV16 + lesions exhibited reduced tumor growth, extended lifespans, and the development of a protective immunological memory. A therapeutic vaccine based on mRNA has the potential to provide a non-invasive alternative to the current standard of care for HPV16 + HSILs, according to researchers. The oncogenic early proteins E6 and E7 are widely recognized targets of HPV16 vaccination strategies. As mentioned, E6 and E7 can be modified with deletions and mutations, respectively, to prevent them from binding to p53 and pRb, thereby eradicating their potential for oncogenesis. A highly efficient IgE leader sequence is encoded in the mRNA of the HPV-16 vaccine developed by researchers. This sequence aids in the expression of a fusion protein that comprises the modified E6 and E7, which are partitioned by a furin cleavage site [121].
According to another research, efforts to fight HPV are still being made via the development of recombinant protein-based vaccinations. The primary capsid protein L1, which may clump together to form VLP and trigger strong immunological reactions, is the protein most often exposed to the immune system. Cervarix and Gardasil, the two primaries approved and commercially available HPV vaccines, now use systems based on pure recombinant VLP. Using E. coli as an example, the HPV L1 protein might be produced in prokaryotic, eukaryotic, insect, plant, and yeast systems, among others. Scientists persist in developing MEV primarily due to its cost-effectiveness, high efficacy, and safety. At present, the most efficacious vaccine against HPV is one based on VLPs; these vaccines can be manufactured using a variety of expression systems. The study centers around a comparison of the recombinant protein expression of L1 HPV-52 using two widely used yeast strains, namely Hansenula polymorpha and Pichia pastoris, both of which have been employed in the industrial production of vaccines. Reverse vaccinology, a bioinformatics technique, was also used by researchers to create alternative MEVs for recombinant protein and mRNA types. Compared to H. polymorpha, P. pastoris exhibited a greater degree of L1 protein expression and production efficiency in a batch system, according to the researchers’ findings. Nonetheless, upon protein induction, both hosts demonstrated stable integration and the creation of self-assembly VLPs. Researchers have developed vaccines that are safe to forecast computationally and have significant immune activation. Additionally, it could work well for manufacturing in a range of expression systems. Large-scale manufacture of the HPV-52 vaccine may be based on this study’s monitoring of the overall optimization parameter evaluation [26].
SQZ-eAPC-HPV (eAPC) is an autologous, HLA-genotype-agnostic, cell-based therapeutic cancer vaccine for HPV that utilizes manufacturing and clinical expertise gained from the SQZ-PBMC-HPV clinical candidate (NCT04084951), according to another study. Cell Squeeze® technology is used to engineer eAPC from peripheral blood mononuclear cells to deliver concurrently five mRNAs encoding for the full-length HPV16 E6 and E7 proteins, CD86, membrane-bound (mb) IL-2, and mbIL-12 cytokines. In addition to minimizing the known toxicity of interleukin treatment, the eAPCs may promote more robust T cell activation and proliferation by promoting co-localized MHC-I antigen presentation (E6/E7), costimulation (CD86), and cytokine signaling (IL-2/IL-12). The effects of multiplexed mRNA-based creation of a cancer vaccine are investigated using eAPC [122].
An extra study demonstrates that infections with HPV-16 are linked to a diverse range of malignancies. Furthermore, it provides strong evidence that the transformative capability of HPV-16 is heavily reliant on the expression levels of the viral oncoproteins E6 and E7. Therapeutic cancer vaccines that possess the ability to induce lasting and targeted immunity against these HPV-16 antigens exhibit significant potential in attaining permanent disease management. Researchers demonstrate that intravenously administered cancer vaccine HPV-16 E7 RNA-LPX, which is composed of immuno-pharmacologically optimized antigen-encoding mRNA, primes and expands antigen-specific effector and memory CD8 + T cells in mice. Immunized mice harbor HPV-positive TC-1 and C3 tumors that are densely infiltrated with HPV-16-specific T cells and activated immune cells; these cells are oriented in a proinflammatory, cytotoxic, and less immune-suppressive manner. Immunization against E7 RNA-LPX induces lasting and comprehensive remission of advancing malignancies. Highly cytotoxic memory T cells in circulation protect tumor re-exposure. In addition, checkpoint blockade rendered anti-PD-L1 refractory tumors susceptible to E7 RNA-LPX immunization. In summary, the findings of the researchers underscore the potential of HPV-16 RNA-LPX in mitigating malignancies caused by HPV [123].
According to different research, CIN, a precursor to CC, is mainly caused by infection with HR-HPV strains. Despite the great efficacy of preventive HPV vaccinations, a significant number of women remain at risk for CIN because of accessibility issues, a lack of knowledge, or personal preferences. Although the loop electrosurgical excision process is a successful therapy for many CIN2/3 patients, it may have serious side effects and does not entirely remove the underlying HPV infection. Thus, non-surgical therapies for the management of high-grade CIN represent an unmet medical need. Nutcracker Therapeutics Inc. has created a novel therapeutic vaccination medication candidate called mNTX-250, which is based on mRNA. It includes the HPV-16 E6/E7 oncoproteins housed in the human cluster of differentiation (hCD1d) scaffold, immunomodulators encapsulated in LNPs, and engineered human LIGHT (hLIGHT) and human interleukin-12 (hIL-12). This study aimed to compare the antigen-specific immune memory induction, pharmacodynamics, and in vivo efficacy of mNTX-250, a murine surrogate for NTX-250, and NTX-010, which includes noncoding mRNA, murine IL-12, and murine LIGHT, against HPV-16 antigens in C3.43 tumors. Reintroduction of TC-1 tumors into tumor-free mice occurred. mNTX-250 therapy, administered in two doses, not only eradicated established B6 C3.43 tumors transformed with HPV16 but also enhanced overall survival, but also generated a substantial population of T cells that are specific to the HPV16 E7 antigen. The tumor-free mice were reintroduced to 105 TC-1 tumor cells, which are B6 mouse lung epithelial cells that have been transformed by HPV16 E6/E7, after their treatment with mNTX-250 or NTX-010. While 6/11 NTX-010-treated tumor-surviving mice had TC-1 tumor development, all mNTX-250-treated mice with completely eradicated C3.43 tumors demonstrated total rejection of TC-1 tumors. When given NTX-010, mice with TC-1 tumor development also produced fewer T lymphocytes specific to the HPV16 antigen. In the C3.43 tumor model, mNTX-250 showed strong anticancer effectiveness and produced immunological memory particular to the HPV-16 antigen that was enough to shield the host against the recurrence of another HPV-16-driven tumor [124] (Table 2).
Advantages and disadvantages of mRNA vaccines
In animal models, mRNA vaccine constructs have been shown in several recent studies to confer protection against an extensive array of infectious agents, including Streptococcus sp, Toxoplasma gondii, Zika virus, rabies, HPV, influenza virus, cytomegalovirus, and Ebola virus [125]. Studies have shown that mRNA-based vaccine formulations may induce strong immune responses in animals, even with a low number of vaccination doses. When given an mRNA–LNP vaccine containing the genetic information for the pre-membrane and envelope (prM-E) glycoproteins of the Zika virus, non-human primates (NHPs) and small animals showed a strong and durable immune response in the form of neutralizing antibodies. This antibody response bestowed sterile immunity against ZIKV infection [126, 127]. In a similar vein, NHPs that were administered an i.m. vaccination using an mRNA vaccine formulated by LNPs and encoding the rabies virus glycoprotein (RABV-G) generated antibody titers that were stable for one year and capable of being boosted [128]. The study found that NHPs who received a single dose of an mRNA-LNP vaccine containing the genetic code for the hemagglutinin glycoprotein of the H1N1pdm09 influenza virus strain produced levels of anti-H1N1-HI antibodies that were at least as high as, if not higher than, the levels considered protective in humans. These antibody levels were also comparable to those produced by a licensed inactivated influenza virus vaccine called fluad [129]. The outcomes of such animal investigations have sparked considerable enthusiasm. Clinical trials are presently underway to evaluate mRNA vaccines against various viral diseases, such as novel coronavirus (SARS-CoV-2), rabies virus, influenza virus, Zika virus, cytomegalovirus, and respiratory syncytial virus. However, apart from the SARS-CoV-2 trial, none of these clinical trials have progressed beyond the initiation phase [19]. As an illustration, a study demonstrated that scientists have created an mRNA-based vaccine targeting the late oncoproteins E6 and E7 of HPV-16. These oncoproteins are expressed exclusively and abundantly in HSILs, a precursor stage to carcinoma in cervical disease. In vitro and in vivo investigations by the researchers established that the translated mRNA induces an antigen-specific adaptive immune response and is functional. Mice harboring HPV-16 + lesions demonstrated tumor growth inhibition, lifespan extension, and the formation of a protective immune memory after vaccination. Given the aforementioned findings and the notable clinical achievements of mRNA vaccines targeting SARS-CoV2, researchers are convinced that their mRNA-based therapeutic vaccine could furnish an alternative non-invasive therapeutic approach to the existing gold standard for HPV-16 + HSILs [121].
The expeditious manufacturing turnover and comparatively low production costs are additional advantages of the mRNA modality. These factors have played a significant role in the triumph of Spikevax® and Comirnaty®, two mRNA vaccines designed to elicit protective immunity against the SARS-CoV-2 Spike protein. Furthermore, the versatility of mRNA synthesis platforms is enhanced by the potential for mRNA to codify for any protein or peptide; consequently, personalized vaccines are no longer an unattainable fantasy. Two of the existing limitations of researchers and other HPV vaccines are the restriction to single genotype-specific antigens (HPV16’s E6 and E7) and the absence of demonstrated overlapping efficacy with other HR-HPV genotypes, which could augment and expand the immune response against HPV lesions. The current vaccine, which targets both E6 and E7, is designed to treat patients with HPV16-associated lesions. In the investigations of Zhou et al., it was observed that patient-derived T cells exhibited an IFNγ response when exposed to putative dendritic cells that express the vaccination protein. This strengthens the confidence of this study regarding the potential for investigators’ vaccine to be translated. The transformation of the C3.43 TME into a cytotoxic signature was an additional significant finding. By analyzing the immunogenicity of spleen cells from vaccinated mice, it was determined that antigen stimulation activated cytotoxic T lymphocytes but not helper T cells. A negligible antibody-dependent cellular cytotoxicity (ADCC) that contributed to the elimination of C3.43 tumor cells cannot be ruled out based on the humoral response that was observed. As E6 and E7 are expressed intracellularly within the transformed epithelium of the cervix, the reported humoral immune response elicited by our vaccine is unlikely to have any practical implications in the form of ADCC implementation. In summary, the immune memory induced by our vaccine, either independently or in conjunction with pre-existing tumor reactivities against C3.43 tumor antigens, resulted in tumor rejection in all complete responders without requiring an additional dose. This finding implies that the Zhou et al. vaccine effectively established a durable barrier against recurrence or reactivation. However, the lack of tumor inhibition observed in C3.43 tumor-bearing mice vaccinated with E1, E2, and E5 indicates that E6 and E7 are primarily targeted by the vaccine-induced immune response, which is more prevalent and protective than other potential immune responses not associated with vaccination. In summary, researchers’ therapeutic vaccine utilizing mRNA to target the oncogenic proteins E6 and E7 of HPV-16 exhibits potential as a candidate for subsequent clinical trial evaluation. Further investigations in larger animal species will provide valuable insights into the clinical translational potential of this vaccine. If efficacious in human subjects, their vaccine may serve as a safer and less invasive substitute for surgical intervention among reproductive-aged women afflicted with HSIL [130].
A plethora of evidence not only demonstrated that mRNA facilitates enhanced transfection efficiency and prolonged protein expression time but also unveiled the primary benefits that distinguish mRNA from DNA. Among the benefits of mRNA are: (1) For mRNA to be functional, it is not required to access the nucleus. Upon entering the cytoplasm, mRNA commences the process of protein translation. DNA, in contrast to mRNA, must first enter the nucleus before transcription into mRNA. DNA is consequently less efficient than mRNA because its functionality is contingent on the disintegration of the nuclear envelope during cell division. (2) Unlike viral and DNA vectors, mRNA expresses the encoding proteins transiently and does not insert itself into the genome. As a consequence of its minimal likelihood of insertional mutagenesis, it presents pharmaceutical companies and researchers with an exceptional safety profile. (3) mRNA synthesis via IVT is straightforward. The procedure is reasonably priced and can be implemented expeditiously across various therapeutic modalities. (4) Furthermore, in theory, mRNA has the potential to encode any protein and can therefore be utilized to treat virtually any ailment [131].
In addition, mRNA has the following benefits over alternative RNA-based HPV-16 vaccines: (1) incorporates antigens E6 and E7 into a solitary polypeptide, obviating the need for concurrent administration of two drug products. (2) administers the vaccine via intramuscular injection, thereby ensuring patient compliance and willingness to receive one or more doses; (3) demonstrates preclinical efficacy at a single and modest dose of 3 µg without exhibiting self-amplification characteristics [130].
In addition to their many benefits and conveniences over conventional vaccines, these have several drawbacks. mRNA vaccines are effective against bacterial pathogens and viral pandemics [132]. Assuming the mRNA vaccine to be clinically safe and efficacious confers numerous benefits. One of the primary benefits is the rate of production. As a result of the synthetic output, neither ova nor cells are necessary for this procedure. By manufacturing vaccines within weeks of discovering the genetic sequence of an immunogen, clinical groups can be formed. mRNA technology enables the rapid synthesis of vaccines targeting multiple targets and facilitates the expression of complex proteins that are otherwise challenging or unfeasible to produce [13, 16]. RNA vaccines are non-contagious and devoid of pathogen mutation risk because they do not contain pathogen particles or inactivated pathogens. Further complicating the administration of the vaccine to cells is the rapid degradation of free RNA within the body. mRNA can induce an inflammatory response due to its rapid disruption following injection. Researchers have discovered that the longevity of mRNA can be extended by encapsulating it with LNPs, which are minute droplets of oil [133]. It can serve as an indicator of an inflammatory response to mRNA while imparting transmission. To mitigate this potential hazard, messenger ribonucleic acid vaccines are encapsulated within larger molecules or encapsulated in particles or liposomes, which aid in the stabilization of the RNA chain. A further drawback of mRNA vaccines is the rarity of adverse effects. Vaccine messenger ribonucleic acid chains induce an unintended immune response because foreign sequences designed by body cells resemble those generated by mammalian cells [134].
Nonetheless, the effective conversion of these molecules into pharmaceuticals is hindered by the following obstacles: (i) the enormous size of mRNA; (ii) its inherent instability and susceptibility to degradation by nucleases; and (iii) its activation of the immune system. While chemical modification of mRNA has helped to address a portion of these obstacles, intracellular delivery of mRNA remains a significant barrier. For mRNA-based therapeutics to be translated into clinical practice, delivery technologies that guarantee mRNA stability under physiological conditions are necessary [135].
Particularly during pandemics, their high potency, low cost, and simplicity of production make them optimal candidates for the prevention and treatment of infectious diseases. The obstacles encountered during in vitro RNA transcription were mitigated to some extent through the utilization of tethered adjuvants or the concurrent delivery of unbound mRNA and adjuvant-tethered RNA [136]. By their generation in a cell-free environment via IVT, mRNA vaccines do not entail any inherent risks [137].
Furthermore, it has been demonstrated that chemical modifications to the mRNA molecule, including altered nucleosides and cap structures, are indispensable for addressing concerns related to immunogenicity, attaining long-lasting stability, and facilitating precise and efficient protein synthesis in vivo [138]. Given its minimal adverse-effect profile and rapid manufacturing process, it emerged as an exceptionally promising candidate for a vaccine [139]. Nevertheless, it is important to note that not everything that appears to be gold is gold, as demonstrated by the vaccine recipients’ reports of severe reactogenicity and a variety of systemic side effects. These adverse effects are causing a change in attitudes towards the vaccine and contributing to vaccine hesitancy [140]. Although they are uncommon, anaphylaxis, antibody-dependent enhancements, and fatalities are the most severe adverse effects. Temperature sensitivity during storage and transport renders it virtually inaccessible to a nation such as India [141].
mRNA-based therapeutics encompass a broad spectrum of applications, including gene modification, protein replacement therapy, and vaccination. The fact that dozens of mRNA-based vaccine candidates are presently undergoing pre-clinical and clinical development demonstrates that mRNA-based vaccine technology holds great promise as a means to create novel prophylactic and therapeutic vaccines against HPV and malignancies associated with HPV. Translation of mRNA-based therapeutics from the laboratory to the bedside is, nevertheless, impeded by a multitude of challenges arising from the enormous size, charge, intrinsic instability, and high vulnerability to enzymatic degradation of mRNA. Hence, the limited applicability of mRNA-based therapeutics persists due to the requirement for enhanced vectors or drug delivery systems. By utilizing sophisticated delivery systems, one can circumvent the inadequate stability, cell specificity, and translational efficacy exhibited by unmodified mRNA. Nonetheless, numerous mRNA vaccine candidates that have undergone clinical testing are formulated devoid of any delivery system. This indicates that additional advancements in delivery systems for mRNA vaccines are necessary. mRNA delivery presently involves lipoplexes and lipid-based nanoparticles preponderantly. In addition, lipid-polymer hybrid nanoparticles and polymers exhibit considerable potential for cost-effectiveness, safety, stability, and transfection efficiency. Prolonged progress in the formulation and delivery of mRNA via diverse nanomaterials has the potential to enhance the applicability of mRNA in the prevention and treatment of HPV and malignancies associated with HPV [135, 142].